There are several kinds of energy that we know of in the universe.
- Kinetic energy is the energy of masses with momentum gathered from potential energy.
- Thermal energy is the energy that is found when atoms of a substance are moving rapidly.
- Solar energy.
- Chemical energy is the energy released from chemical bonds when those bonds are broken or created.
- and several others…
But the most common sort of energy we’ll be talking about in robotics is electrical energy.
In class we discussed many sources of electrical energy. Name three different sources of electricity other than batteries that we see in everyday use?
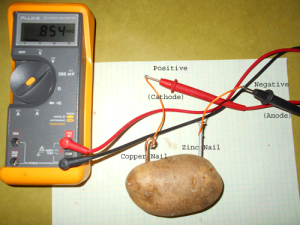
We demonstrated the chemical energy that’s associated with batteries as a source of energy by making a potato battery.
When we connected a copper wire and a zinc coated nail into the potato and measured the voltage we found that we created a little less than a volt of electricity but it only flowed at a rate of less than 1 milliamp.
To figure out how a potato and a couple of metal nails can make electricity it was necessary for us to explore some atomic physics.
According to current theory, all atoms are comprised of three fundamental parts
- Neutrons
- Protons (positively charged particles)
- Electrons (negatively charged particles)
The Neutrons and Protons combine into what we call the nucleus of the atom and the electrons are thought to orbit this nucleus in layers called shells. In neutrally charged atoms the number of electrons should equal the number of protons. In class I showed you a periodic table which organized all the atoms we know of in order of how many protons they had.
 The outermost shell of any atom is sometimes called the valence shell. Electrons from this layer can be shared with another atom. When this happens we create a chemical compound and the shared electron is called a covalent bond. In some elements this outer layer is short one electron or only has one electron, making the atom very reactive!
The outermost shell of any atom is sometimes called the valence shell. Electrons from this layer can be shared with another atom. When this happens we create a chemical compound and the shared electron is called a covalent bond. In some elements this outer layer is short one electron or only has one electron, making the atom very reactive!
The copper and zinc (I’ve highlighted them in blue) are numbers 29 and 30 on the periodic table. The Potato has a small quantity of phosphoric acid in it that reacts with the copper and the zinc to release some electrons by oxidizing (rusting) the zinc and reducing (the opposite of oxidizing) the copper. This places more electrons on the zinc and fewer electrons on the copper. When we connect them to a drain (a light for example) the electrons flow from the electron-rich zinc to the electron poor copper.
The main point of most technologies is to do work. Work is using a force to move something or to keep it from moving. Remember from your sailboats that force is a vector quantity; It operates in a specific direction. In most cases, the work done will also be in specific direction. In class we showed the work done by rolling a steel ball down a ramp and across the floor. We also talked about the torque of the motors on our BOE-BOT’s axle. Finally we discussed how, because force is a vector quanity, we can divide the momentum in a rolling steel ball between other items and change its direction and speed. Research and write a page or more in your journals about vectors and how they can be added.
Power is not the same thing as energy. When energy gets used to do something (work) we call that power. It is usually measured in terms of the rate at which the energy is used to accomplish one unit of work. Energy only exists when its flowing.
For our potato battery we would multiply the amperage (current) and the voltage together to get the power of our potato battery. Although it had enough voltage to power a motor it would take several dozen potatoes before you had enough current to power a small motor.
In class we discussed how to string together potatoes to increase voltage or to increase current. Can you remember how we did that?
Want to know more
Leave a comment